Maillard Reaction
Posted on Categories Discover Magazine

Canadian Bacon Donut Complimentary of Portobello Cafe in Whistler, Canada. This donut provides many examples of the Maillard reaction. When frying the donut batter, high temperatures promote browning of the dough and also impart crispiness. Secondly, the bacon! the flavors in bacon are the result of Maillard reaction products. The browning of the bacon creates and releases flavnoids. Photo Credit: Steven Du
Guest post by Steven Du
The flavor reaction. What makes bread crust brown and tasty? What makes the smell of searing meat so savory and delicious? How can grill marks and black crust on meats, supply such a flavor punch?
Three words: the Maillard reaction. This simple reaction leads to thousands of flavonoids that impart food with flavors that make us come back for more every time. The essential components of the Maillard reaction are protein and sugars that lead to flavonoids that make food delicious.
Several factors are important for the Maillard reaction; however, the most important factor is heat. The origins of the Maillard reaction can be traced to a mistake of our ancestors to roast meat on a flame [1]. This simple technique of roasting led to an explosion of culinary techniques and a quest to find flavors to satisfy our desires of delicious foods.
The Maillard reaction requires two other important factors besides heat: protein and sugars. To kick-start the reaction, you need about 300 degrees Fahrenheit of heat. Heat beyond 300 degrees Fahrenheit is required because the moisture and water on the surface of proteins need to evaporate, which happens around 212 degrees Fahrenheit. The temperature must be beyond 300 degrees Fahrenheit to activate the reaction since it is non-enzymatic. Advice for a budding chef attempting to make a delicious steak dinner is to salt for 30 minutes prior to release more moisture and allow the steak to be dryer. [3] By drying the meat, the proteins are exposed to higher temperatures earlier enabling the tasty Maillard reaction to take place. The other important components of the Maillard reaction are the proteins and the sugars. However, an abundance of sugar leads leads leads to caramelization due to the lack of protein and amino groups to initiate the Maillard reaction. Caramelization leads to a sweet nutty flavor and brown coloring. By contrast, Maillard reactions result in the development of additional flavors with the addition of the amino groups from proteins, creating a multitude of flavonoids and aromas that blend together together to form a sweet or savory dish.
The basic chemistry in this reaction happens between amino acids and reducing sugars. The carbonyl group of the sugar reacts with the amino group of the amino acid and produces water and other intermediates, which undergo multiple rearrangements [1]. These rearrangements can be from the amadori rearrangement to short-chain hydrolitic fission1 which lead to deoxyhexodiulose and other aroma, flavor, and color compounds as seen in figure 1. The more we “brown” the item, the more diverse the flavonoids that come out. However, overdoing the reaction can also lead to toxic byproducts, such as those found in black and burnt foods [1]. Melanoidins are common byproducts from the Maillard reaction people are familiar with, which are the browning pigments seen on meats and breads which are the results of the Maillard reaction.
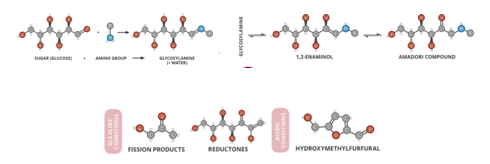
Figure 1. Maillard Reaction Scheme and possible products. Photo Credit: Compound Interest
From the basic meat we cook on the stove, to the browning of bread, there is a variety of recipes that utilize the complex yet pleasuring and delectable Maillard reactions to create delicious foods . The Maillard reaction not only shaped the development of food, but it also revolutionized how we look at food.
References:
- Martins, Sara, et al. “A Review of Maillard Reaction in Food and Implications to Kinetic Modeling.” Food Science and Technology, 2011, www.researchgate.net/profile/Sara_Martins3/publication/222682804_A_review_of_Maillard_reaction_in_food_and_implications_to_kinetic_modelling/links/0fcfd51140ffcf26ff000000.pdf.
- Tamanna, Nahid, and Niaz Mahmood. “Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition.” International Journal of Food Science, Hindawi Publishing Corporation, 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4745522/.
- Schulze, Eric. “An Introduction to the Maillard Reaction: The Science of Browning, Aroma, and Flavor.” Serious Eats, 13 Apr. 2017, www.seriouseats.com/2017/04/what-is-maillard-reaction-cooking-science.html.