A New Freeze-dried Polio Vaccine Could Help Finally Eradicate the Disease
Posted on Categories Discover Magazine
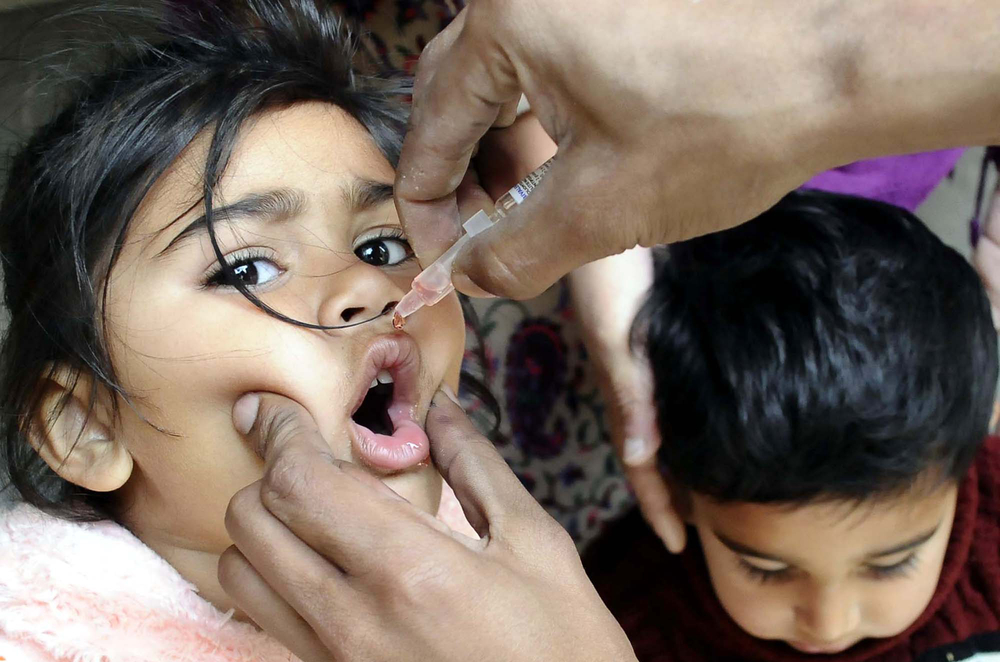
A health worker administrates polio-vaccine drops to a child during an anti-polio immunization campaign on March 09, 2017 in Rawalpindi, Pakistan. (Credit: Asianet-Pakistan/Shutterstock)
Polio once paralyzed more than 350,000 people each year worldwide. Today, vaccines have dropped the number of reported cases to just 407 in 2013, according to the CDC.
But the disease still lurks in developing countries because vaccine storage and transport requires refrigeration. Now, scientists find freeze-drying a polio vaccine keeps it stable for as long as four weeks even in hot temperatures. The stability could enable vaccine distribution that could lead to the end of the disease.
“No matter how wonderful a drug or vaccine is, if it isn’t stable enough to be transported, it doesn’t do anyone much good,” Woo-Jin Shin, a virologist at the University of Southern California in Los Angeles and co-author of the new research, said in a statement.
Polio Paralysis
Poliovirus causes the malady known as polio, a highly infectious disease that often starts out with flu-like symptoms but can lead to permanent paralysis. The condition becomes deadly when an infected individual’s breathing muscles become immobile.
In the U.S. and other developed countries, vaccines eliminated the disease, yet the virus still has strongholds in Afghanistan, Nigeria and Pakistan. Storing the vaccine depends on keeping it cold. But refrigeration is not reliable or available in many developing countries. Jae Jung, a microbiologist and Shin’s advisor, wanted to make a shelf-stable vaccine.
Suitable Stability
Jung and his research team first isolated an injectable polio vaccine. Then, like bakers playing with various amounts of sugar, milk and butter to make the perfect frosting, the researchers tried many ways to stabilize the vaccine. Among other things, they tested how much moisture to extract from the vaccine and the concentration of different stabilizers to add.
The scientists discovered they needed to remove a lot of moisture from the vaccine. The moisture content of the winning formulation was only 0.77 percent, the team reports today in the journal mBio.
The researchers then put their discovery to the test. They stored the freeze-dried vaccine for four weeks at 37 degrees C (that’s 98.6 degrees F). When the scientists vaccinated mice with the unrefrigerated vaccine, the rodents’ immune systems responded as though they had received the refrigerated vaccine. The new vaccine also protected the mice from infection with the poliovirus as well as a commercial vaccine.
Health centers only have so much space to store vaccines, but if scientists can extend the medicine’s shelf life to perhaps three or even 12 months, then the vaccine supply chain could get rid of the need for cold storage and bring the world one step closer to polio eradication.